Company: Suvoda, a global clinical trial technology company
Platform: Mobile AppTimeline: 2020-2022
2020 Research | 2021 MVP | 2022 Release to Priority Clients | 2023 Open Market (quarterly release cycle)
2020 Research | 2021 MVP | 2022 Release to Priority Clients | 2023 Open Market (quarterly release cycle)
Team: Project Manager, Product Owner, UX Designer, Engineering Team, Stakeholders, Lead Scientists
Role: UX Designer
Tools Used: Figma, Figjam, Android Studio
Design Task
Setting out as the Lead UX Designer on this project, my goal was to create a user-centric, mobile-first application catering to patients (and their caregivers) participating in a clinical drug trial focused on collecting data related to their clinical trial.
The app's primary objectives were to enhance user engagement (increase patient compliance), reduce friction (for both patient and client/study), and provide a efficient experience achieved by accessibility and an intuitive interface.
Background
At the beginning of this design project, I needed a lot of background information about Suvoda, clinical drug trials, the software needed for these trials, and all the regulation around Patient Privacy and Data Privacy. I entered this Discovery phase with curiosity because there was so much I didn't know. Like...
What is eCOA?
eCOA stands for Electronic Clinical Outcome Assessment. It is a term used to describe the collection of patient-reported outcome (ePRO), clinician-reported outcome (eCLinRO), observer-reported outcome (eObsRO), and performance outcome (ePerfO) data in clinical trials using electronic devices.
eCOA technology offers several benefits, including improved data quality, more efficient data collection, and the ability to capture and manage patient or caregiver reported outcomes.
Why design a mobile app for eClinical trials?
It is needed in clinical trials to ensure the accuracy and completeness of data, minimize the risk of errors, and deliver more accurate outcome assessment data.
It solves issues from the previous standard of paper collection such as manual errors and delayed data entry pain points.



My Process
Discover
Integrating user research, market analysis, and quantitative user data into the UX design process is crucial to establish a design that is firmly grounded in a comprehensive understanding of user needs, preferences, and pain points.
We conducted interviews and surveys with elderly adults in clinical trials to understand their challenges, preferences, and technological comfort levels.
The following research on the wider app usage for Heath needs in our target demographic reinforced our company's individual findings.
As people age, a number of changes commonly happen to their vision. Many older adults use reading glasses or opt for much larger font sizes when given the option. Overall, color contrast should be increased in websites and apps that cater to older adults.
My goal for this eCOA solution was to design a simple, intuitive interface with larger text and buttons for ease of use with high visual contrast.
Competitive analysis on apps in the same market which were the following: ERT, Signant Health (Bracket and CFR), and Medidata. While many of these apps held strengths of well known names in the clinical study space, it means these apps also were more of a legacy system with the screens looking very full and not designed in a mobile first approach, or without consideration to low vision users.
This analysis informed me as the lead UX designer about industry benchmarks and helped me to make informed decisions to create a more competitive and user-centric design.
Define the Problem
Before I could jump into designing the actual pages, it was important to define success for this project. To do so, I made a note of high value requirements that needed to be considered in order to make this project a success: accessibility and intuitive interface.
Personas
The primary objective of personas in the initial stages of product discovery aimed to to identify the core needs of the target audience, guiding the creation of user profiles and informing sample recruitment for user interviews, which were unfortunately delayed due to Covid restrictions.
Mary is the main persona, our elderly patient in clinical drug trial and is supported by her son Dave who is her primary caregiver as her husband passed many years ago.
In a brainstorm session I facilitated with senior stakeholders, the focus was on collaborative problem-solving and ideation, specifically business goals, capturing lifecycle of the new concept of a "Suite of Products" and how they all flow together, painpoints and opportunities.
Together, we generated ideas and mapped out potential solutions on the whiteboard, fostering creativity and alignment between business objectives and user needs. Throughout the session, I encouraged active participation, asked probing questions while capturing key takeaways to inform the design direction.
User stories informed the scenarios for which to the start designing the task flows. Deep collaboration between the Platform Project team and UX informed how best to use the authentication tools while still centering the user experience.
Design and Iteration
Features
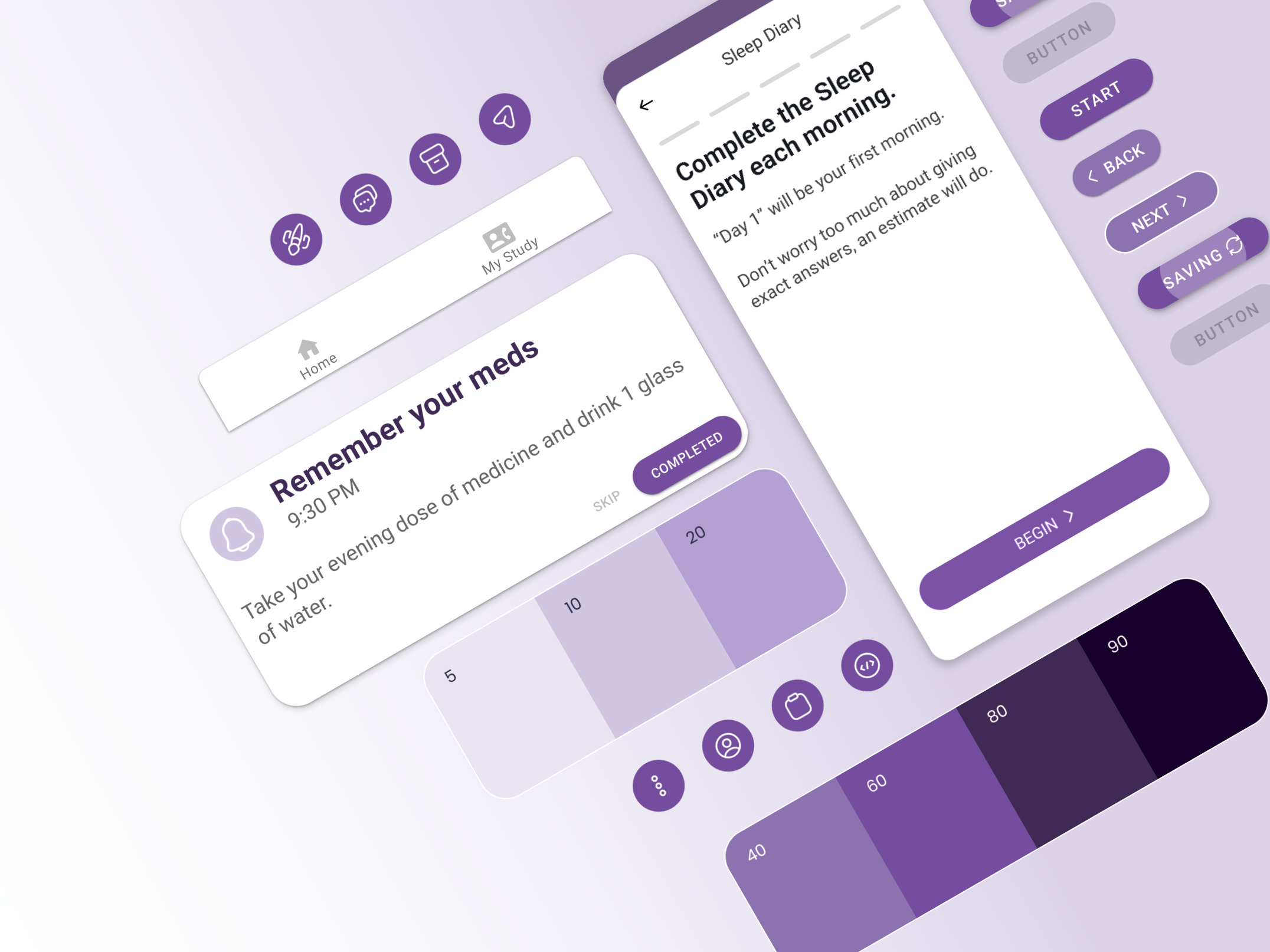
My Day: Customizable dashboards displaying relevant metrics (such as available assessments, medication reminders, and appointments) were designed.
My Study: Collection of information specific for the user's clinical trial including clinic location, hospital map and location, study information, and secure messaging functionality allowing direct interaction between users and healthcare providers, facilitating quick consultations or support, plus a repository of signed consent documents.
Assessments: Intuitive interfaces for entering and tracking data and visual cues for easy comprehension.
Results and Impact
In keeping usability and accessibility as main priorities through out the entire process, I created a list of requirements in my designs, next developers who specialized in accessibility implemented the components and screens. And finally the Suvoda eCOA solution was recognized as earning high marks in patient usability as found by RWS Study.

Open Screen for Evening Diary

Usability Workshop

NRS Scale for Evening Diary

Reflection
The success of the newly expanded Suvoda Suite of apps under my UX guidance has culminated in a very successful 2023 year as the company announced a 30% growth driven by strong customer adoption of new products.
This eCOA mobile app lives within the Suvoda Suite of apps and is delivered on a single platform to help guide those in complex clinical trials through novel science.